BIA-ALCL
In 2011 the FDA noted that there was an association between breast implants and anaplastic large cell lymphoma (BIA-ALCL). At the time there was very little information about this topic and over the last few years both the FDA and plastic surgery societies have been extensively involved in learning about this rare cancer. To date approximately 600 cases have been reported worldwide.
What is BIA-ALCL? First of all, it is not a breast cancer, but rather a lymphoma, a cancer of the T-cell of the immune system specifically a non-Hodgkin’s lymphoma. This lymphoma grows in the capsule, or scar tissue (see Figure One) which surrounds the breast implant. In some cases it can spread throughout the body.
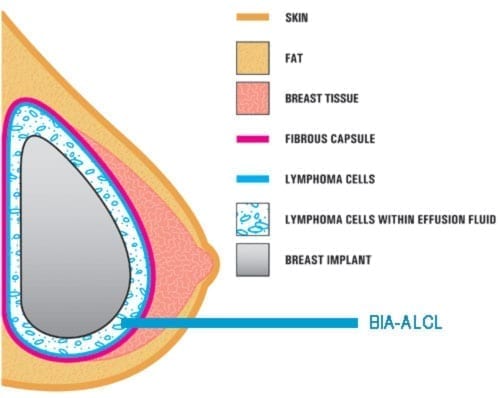
Figure One : BIA-ALCL adopted from Thompson, et al 2010 and FDA, 2019
The American Society of Plastic Surgery (ASPS) and the American Society of Aesthetic Plastic Surgery (ASAPS) have been proactive in educating plastic surgeons about the disease as well as collecting data and assisting in worldwide research in order to learn about this disease and spread knowledge. The level of involvement by our societies with this newly diagnosis and scary entity is wonderful and unprecedented.
Over the last few years more and more cases have been reported. As awareness of the entity has spread, this has helped to diagnose more cases. Initially we were told that there was a one in a million chance of a patient developing ALCL. Current data (as of Spring 2019) shows a risk of somewhere between 1:3800 and 1:30000 risk of developing ALCL.
Most patients are diagnosed with ALCL between 2 years and 28 years after augmentation with half of the patients diagnosed with ALCL have been within 7-8 years of their augmentation. The vast majority, if not all of the cases have been associated with textured breast implants. The fill of the implants, saline versus silicone does not matter.
What are the symptoms? Any patient with a late onset seroma, or fluid collection that develops around the implant years after initial augmentation needs to be checked for ALCL. Masses or swelling adjacent to a breast implant require evaluation. Pain, asymmetry, firmness or lumps in the breast or axilla (armpit) are all reasons to get seen by your plastic surgeon.
There are several theories to why ALCL develops. In the predominant theory, the texturing of the implant is believed to allow for a greater surface area for bacterial contamination, biofilm production, and chronic inflammation which leads to the immune system going awry. In another theory mechanical irritation from the texturing causes chronic inflammation. Finally another theory is that the microscopic shedding of silicone from the textured surface creates chronic inflammation.
The good news is that treatment of ALCL is very successful when the disease is diagnosed early. Early detection is very important, because there have been a few deaths reported from advanced disease. Usually surgical removal of the implant and capsule is curative. Sometimes further treatment such as chemotherapy, radiation, or further surgery may be necessary. Unfortunately there are 9 known deaths from ALCL in the United States (as of early 2019) so treatment is best when started early.
Since texturing of implant surfaces is the significant risk factor for ALCL, one might ask should these implants be removed? In April of 2019 the FDA asked Allergan to remove their textured breast implants from the American market. They did not ask Mentor or Sientra to remove their textured devices. The differences between these implants were the degree of texturing present in Allergan’s implant. The vast number of ALCL cases had been associated with Allergan’s texturing process, thus the FDA acted to stop their use. Texturing is the predominant risk factor and different manufacturers use different texturing manufacturing processes. It is interesting that some texturing is more of a risk than others!
There is no current recommendation (March 2020) by the FDA or any American Plastic Surgery Society to prophylactically exchange textured breast implants to smooth breast implants. The only known case of ALCL being reported to the FDA with a smooth implant was later amended as an inaccurate report. They recommend awareness and education as paramount. . Even though BIA-ALCL is a rare entity, he believes smooth breast implants can deliver a beautiful, natural result for the vast majority of patients.
We will continue to learn about BIA-ALCL as more cases are undoubtedly treated over the next several years recommendations will continue to change. Please note this website page was published in Spring 2020 and newer information may be available. For now, patient education is paramount and if there is any change in their breast appearance or feel, patients need to see their plastic surgeon. Should you have any questions please do not hesitate to call the office or schedule a visit with Dr. Sorokin in Philadelphia.